For adults with schizophrenia or bipolar I or II disorder,
IGALMI IS A SUBLINGUAL FILM FOR THE ACUTE TREATMENT OF AGITATION1
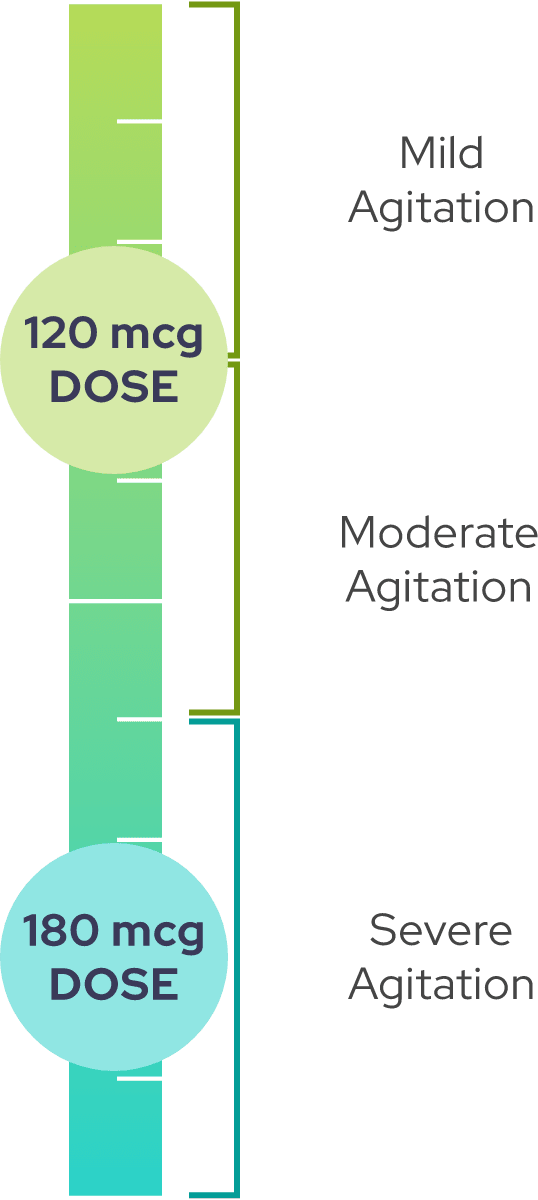
Two dosage strengths are available to treat the
spectrum of
agitation
For adults <65 years of age:

-
Lower dosages are recommended for geriatric patients (≥65 years of age) or those with hepatic impairment
-
If agitation persists after the initial dose, up to two additional doses may be administered at least two hours apart
-
Assess vital signs, including orthostatic measurements, prior to the administration of any subsequent doses due to risk of hypotension
-
Additional half-doses are not recommended in patients with systolic blood pressure (SBP) less than 90 mmHg, diastolic blood pressure (DBP) less than 60 mmHg, heart rate (HR) less than 60 beats per minute, or postural decrease in SBP ≥20 mmHg or in DBP ≥10 mmHg
-
Please see the full Prescribing Information for all recommended dosing instructions.

SUBLINGUALLY (under tongue)
wait 15 minutes to eat or drink
OR

BUCCALLY (behind lower lip)
wait 1 hour to eat or drink
The patient should not chew or swallow the film.
Supervision and monitoring required for IGALMI1
-
IGALMI should be administered under the supervision of a healthcare provider
-
A healthcare provider should monitor vital signs and alertness after IGALMI is administered to prevent falls and syncope
-
After IGALMI administration, patients should be adequately hydrated and should sit or lie down until vital signs are within normal range. If a patient is unable to remain seated or lying down, precautions should be taken to reduce the risk of falls. Ensure that a patient is alert and not experiencing orthostatic hypotension or symptomatic hypotension prior to allowing them to resume ambulation
Please see the accompanying full Prescribing Information for complete preparation and administration instructions (ie, adjustments for patients with hepatic impairment, optional half-dosing, etc).
During clinical trials, all patients self-administered IGALMI under the supervision of an HCP.1-3
IMPORTANT SAFETY INFORMATION
INDICATION
See More
Collapse
IMPORTANT SAFETY INFORMATION
IGALMI is self-administered under the supervision of a healthcare provider. A healthcare provider should monitor vital signs and alertness after IGALMI administration to prevent falls and syncope.
WARNINGS AND PRECAUTIONS
Hypotension, Orthostatic Hypotension, and Bradycardia: IGALMI causes dose-dependent hypotension, orthostatic hypotension, and bradycardia. In clinical studies with IGALMI, patients were excluded if they had treatment with alpha-1 noradrenergic blockers, benzodiazepines, other hypnotics or antipsychotic drugs four hours prior to study drug administration; had a history of syncope or syncopal attacks; SBP < 110 mmHg; DBP < 70 mmHg; HR < 55 beats per minute; or had evidence of hypovolemia or orthostatic hypotension. Because IGALMI decreases sympathetic nervous system activity, hypotension and/or bradycardia may be more pronounced in patients with hypovolemia, diabetes mellitus, or chronic hypertension, and in geriatric patients. Avoid use of IGALMI in patients with hypotension, orthostatic hypotension, advanced heart block, severe ventricular dysfunction, or history of syncope. After IGALMI administration, patients should be adequately hydrated and should sit or lie down until vital signs are within normal range. If a patient is unable to remain seated or lying down, precautions should be taken to reduce the risk of falls. Ensure that a patient is alert and not experiencing orthostatic hypotension or symptomatic hypotension prior to allowing them to resume ambulation.
INDICATION
IGALMI is indicated for the acute treatment of agitation associated with
schizophrenia or bipolar I or II disorder in adults.
Limitations of Use: The safety and effectiveness of IGALMI have not been established beyond 24 hours from the first dose.
QT Interval Prolongation: IGALMI prolongs the QT interval. Avoid use of IGALMI in patients at risk of torsades de pointes or sudden death, including those with known QT prolongation, a history of other arrhythmias, symptomatic bradycardia, hypokalemia, or hypomagnesemia, and in patients receiving other drugs known to prolong the QT interval.
Somnolence: IGALMI can cause somnolence. Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or operating hazardous machinery, for at least eight hours after taking IGALMI.
Risk of Withdrawal Reactions, Tolerance, and Tachyphylaxis: IGALMI was not studied for longer than 24 hours after the first dose. There may be a risk of physical dependence, a withdrawal syndrome, tolerance, and/or tachyphylaxis if IGALMI is used in a manner other than indicated.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension.
DRUG INTERACTIONS
Drugs That Prolong the QT Interval: Avoid use. Concomitant use of drugs that prolong the QT interval may add to the QT-prolonging effects of IGALMI and increase the risk of cardiac arrhythmia.
Anesthetics, Sedatives, Hypnotics, and Opioids: Concomitant use may cause enhanced CNS-depressant effects. Reduction in dosage of IGALMI or the concomitant medication should be considered.
USE IN SPECIFIC POPULATIONS
Hepatic Impairment and Geriatric Patients (≥65 years old): A lower dose is recommended in patients with hepatic impairment and geriatric patients. See the full Prescribing Information for the recommended dosage depending on the agitation severity.
Please see full Prescribing Information.
To report SUSPECTED ADVERSE REACTIONS, contact BioXcel Therapeutics, Inc. at 1-833-201-1088 1-833-201-1088 or medinfo@bioxceltherapeutics.com, or FDA at 1-800-FDA-10881-800-FDA-1088 or www.fda.gov/medwatch.
References:
1. IGALMI. Package insert. BioXcel Therapeutics, Inc.; 2022. 2. Data on file. BXCL501-301 CSR (SERENITY I). BioXcel Therapeutics, Inc.; January 2021. 3. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799 4. Data on file. BXCL501-302 CSR (SERENITY II). BioXcel Therapeutics, Inc.; January 2021.



