In adults with schizophrenia and bipolar I or II disorder,
THE SERENITY I AND II TRIALS EVALUATED IGALMI FOR THE ACUTE TREATMENT OF AGITATION1
Identically designed, randomized, placebo-controlled, parallel-group, fixed-dose trials comparing placebo to two active arms
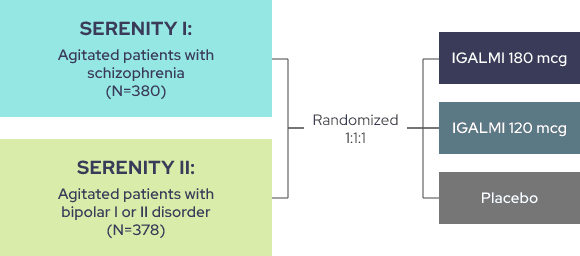
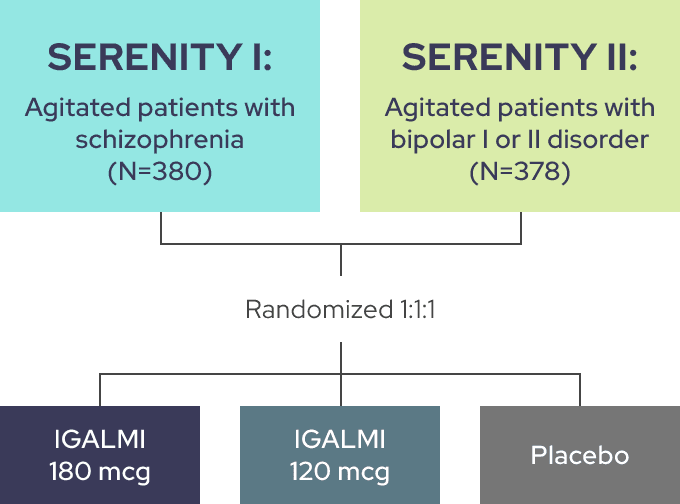
Trial endpoints:
-
Primary endpoint: Change from baseline in the Positive and Negative Syndrome Scale-Excited Component (PEC) score at 2 hours following initial dosing
-
Key secondary endpoint: Time to effect onset as measured by the change from baseline in the PEC score following initial dosing
PEC is a validated, investigator-rated tool to assess agitation severity across five main items1,2
Excitement
• Uncooperativeness
• Tension
• Poor impulse control
• Hostility
Each item is rated on a scale of 1 (absent) to 7 (extremely severe). The total PEC score is calculated by taking the sum of the individual items.1-3
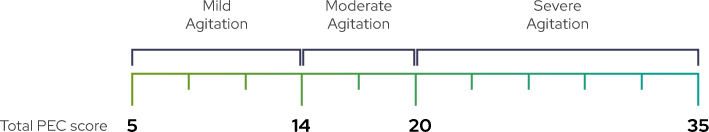
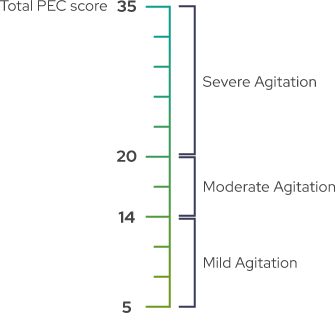
A PEC score of ≥14, with at least one individual item score of ≥4, was required for enrollment1
As shown by baseline characteristics, a broad range of patients were included1
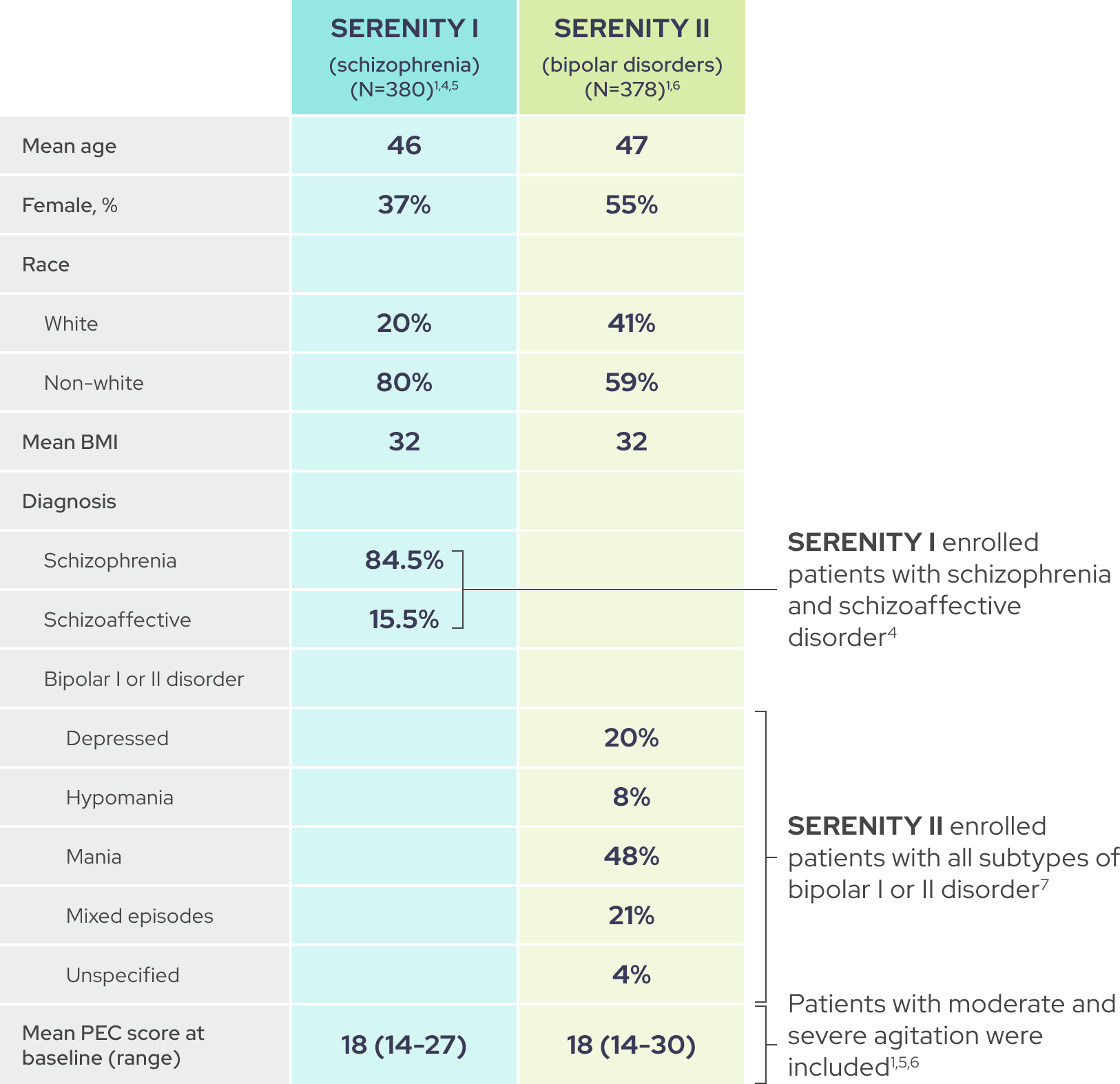
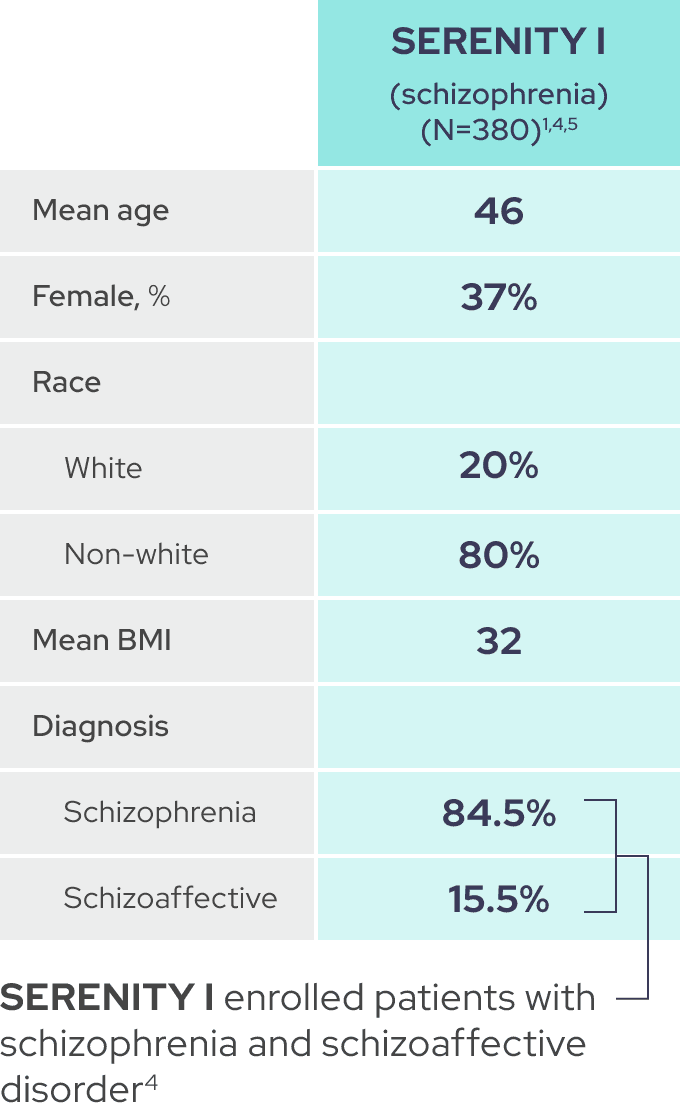
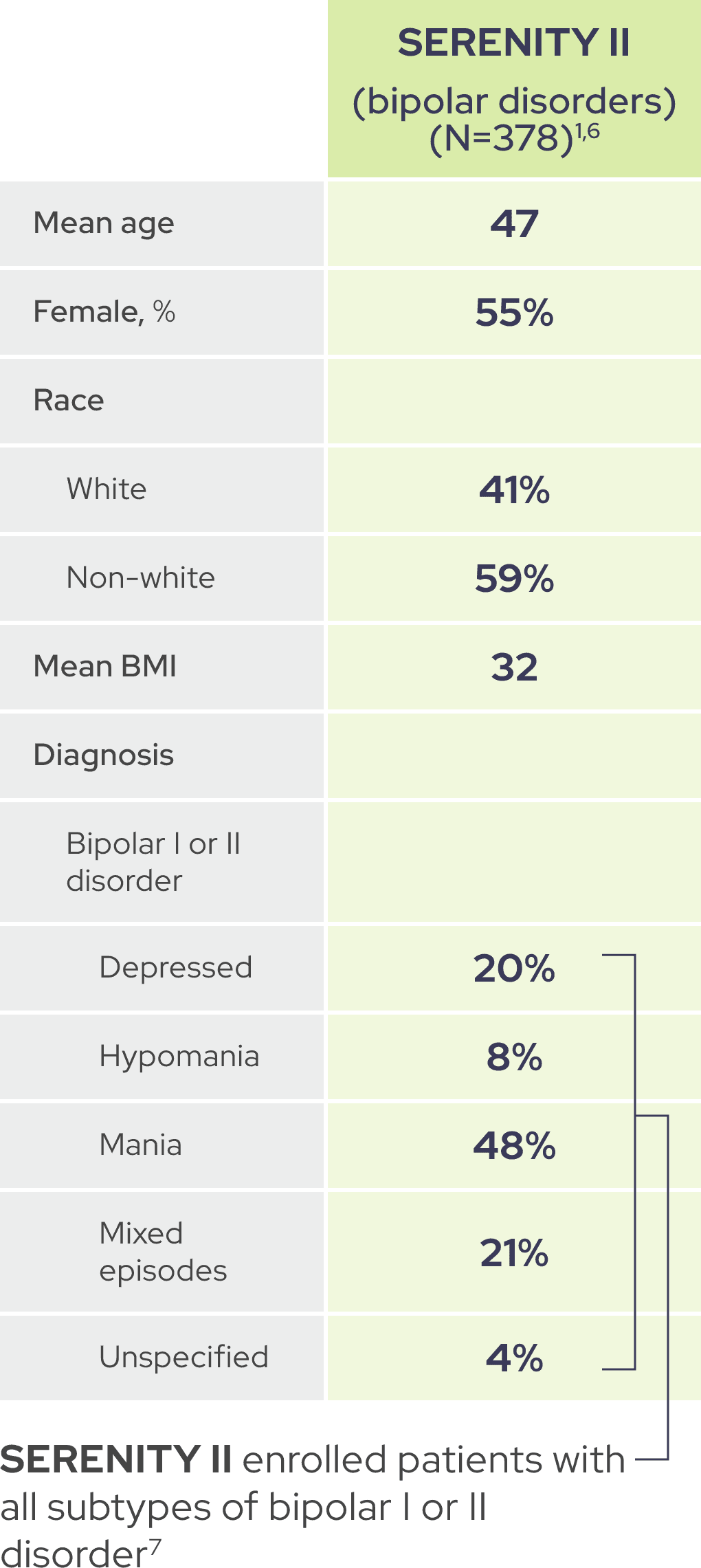
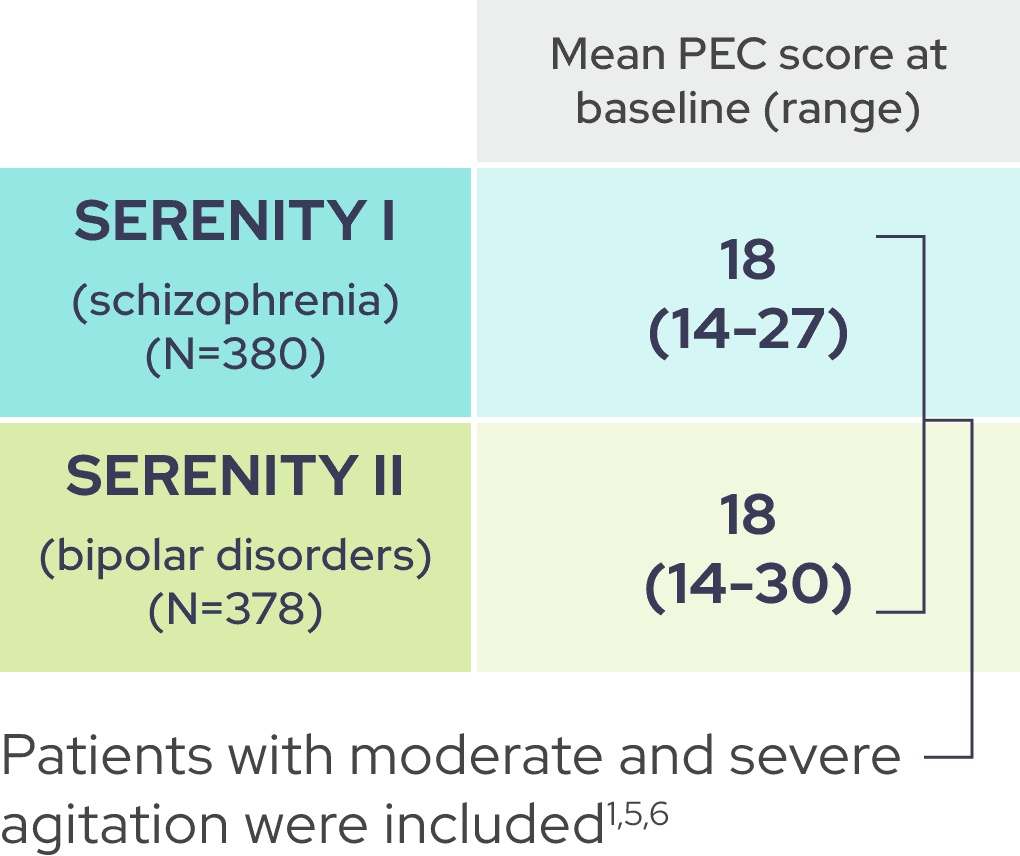
In each trial, demographic and baseline disease characteristics were comparable across placebo and IGALMI treatment arms.1,4,6

